So carbon shares 2 with one oxygen atom and 2 with the other oxygen atom. The carbon is the central atom and all the atoms are attached to the carbon except for a hydrogen bonded to an oxygen.

Of2 Lewis Structure How To Draw The Lewis Structure For Of2 Youtube
Draw the electron dot structure of formic acid H2CO2.

. Up to 24 cash back Draw Lewis structures AND predict the molecular geometry of the following compounds or polyatomic ions. Iodine What do you observe about the three structures. The oxygens in NO_2- are attached to the nitrogen.
Draw the electron dot structure for the hydro- gen carbonate ion HCO. Draw the electron dot structure for the hydrogen carbonate ion HCO_3-. A step-by-step explanation of how to draw the MgO Lewis Dot StructureFor MgO we have an ionic compound and we need to take that into account when we draw th.
A CaOan ionic compound b HBr c N2 Base your answers to questions 2 and 3 on the information below and on your knowledge of chemistry. Lewis Electron Dot Structure for the molecule. Using electron dot structures draw at least two resonance structures for the nitrite ion NO_2-.
A substance that donates a pair of electrons is defined as a. Draw Lewis dot structures showing the formation of an ionic compound from each of the following combinations of elements show electron transfer and resulting charges. Electron-dot structure of is as follows.
Draw the electron dot structure for each molecule. So first of all um if. Lewis structures also known as electron dot structures are named after Gilbert N.
So Al can accept a pair of electrons to complete its octet. H 2 O 2 b. H2S 2 is a subscript.
Lewis Electron Dot Structure of the molecule. Each substance only contains single covalent bonds. A molecular formula shows how many atoms of each element a substance contains.
32 valence electrons or 16 pair C is in center with 4 Cls aroundthis uses 8 electrons or 4 pair. Identify polar covalent bonds by assigning slightly positive and. The bonds between the following pairs of electrons are covalent.
The question here gives us um two substances a being um f three s s and F and B being ch three c double bond C c 02 minus. They all look similar with respect to the electrons. Chapter 8 Problem 49A is solved.
If you cannot do either then explain in words exactly where the dots would be around the atoms in the molecules. CO carbon monoxide The carbon atom has a valency of 4. Each of the following molecules contains one or more multiple covalent bonds.
Draw electron dot structures for each molecule. Draw an electron dot structure for each. Carbon is the central atom and hydrogen is attached to oxygen in this polyatomic anion.
Or you will need to use the rich text to type them. Lewis who described them in a 1916 article titled The Atom and the Molecule Lewis structures depict the bonds between atoms of a molecule as well as any unbonded electron pairs. A step-by-step explanation of how to draw the OF2 Lewis Dot Structure Oxygen difluorideFor the OF2 structure use the periodic table to find the total numb.
We still have 12 pairplace 3 pair on each terminal Cl atom. Draw electron dot structures for the following molecules which have only single covalent bonds. Draw plausible electron dot structures for the following substances.
How many electron domains are around the central atom. Step 5 of 5. Draw plausible Lewis structures to represent this.
The electron dot structure of is shown below. You will need to draw them by hand and upload an image. So it wants to say draw the election dot structure.
Each bond contributes two electrons. Total number of electrons around the Al atom are 6 from the three bonds. Up to 24 cash back 1.
Carbon is the cen- tral atom and hydrogen is attached to oxygen in this polyatomic anion. Pearson Chemistry 1st Edition. Draw the Lewis dot structure for eqXeI_4 eq and answer the following questions.
A substance that accepts a pair of electrons is defined as a Lewis acid. Draw the electron dot structure of the poly- atomic thiocyanate anion SCN. A step-by-step explanation of how to draw the NOCl Lewis StructureFor the NOCl structure use the periodic table to find the total number of valence electron.
Oxalic acid H 2 C 2 O 4 is a poisonous substance found in uncooked spinach leaves. Problem 65 Medium Difficulty. View this answer View this answer View this answer done loading.
How must the electronegativities of two atoms compare if a covalent bond between them is to be polar. 1Draw an electron-dot diagram for each of the following substances. The following molecules have single covalent bonds.
CCl4 Tally the valence electrons C 1 4 4 Cl 4 7 28 Total. Step 2 of 5. Draw the electron dot structures for the following substances.
Draw the electron dot structure of the hydroxide ion OH-. The formulas and the boiling points at standard pressure for ethane methane methanol and water are shown in the table below. Step 3 of 5.
H 2 S d. Incidentally oxalic acid is the reason your teeth feel a little cleaner after eating spinach. View a sample solution.
Up to 256 cash back Draw plausible electron dot structures for the following substances. Step 4 of 5. Hence two double bonds are formed.
Each substance contains only single covalent bonds. If oxalic acid has a C-C single bond and no C-H bond draw its electron-dot structure. Each substance contains only single covalent bonds.
An oxygen atom has 6 valence electrons and a carbon atom has 4. You can draw a Lewis dot structure for any covalent molecule or coordination. Using electron dot structures draw at least two resonance structures for the nitrite ion NO-.

I2 Lewis Structure How To Draw The Dot Structure For I2 Youtube
![]()
Draw Lewis Structures For The Following Compounds And Determine The Shape For Each Molecule A Hcn B So3 C Nh4 D Sncl4 Study Com

Ni3 Lewis Structure How To Draw The Dot Structure For Ni3 Nitrogen Triiodide Youtube

Solved 46 Describe The Difference Between An Ionic And A Chegg Com

Answer Ionic It Has A Very High Melting Point That Is Characteristic Ppt Video Online Download

Solved 47 How Many Electrons Do Two Atoms In A Double Chegg Com
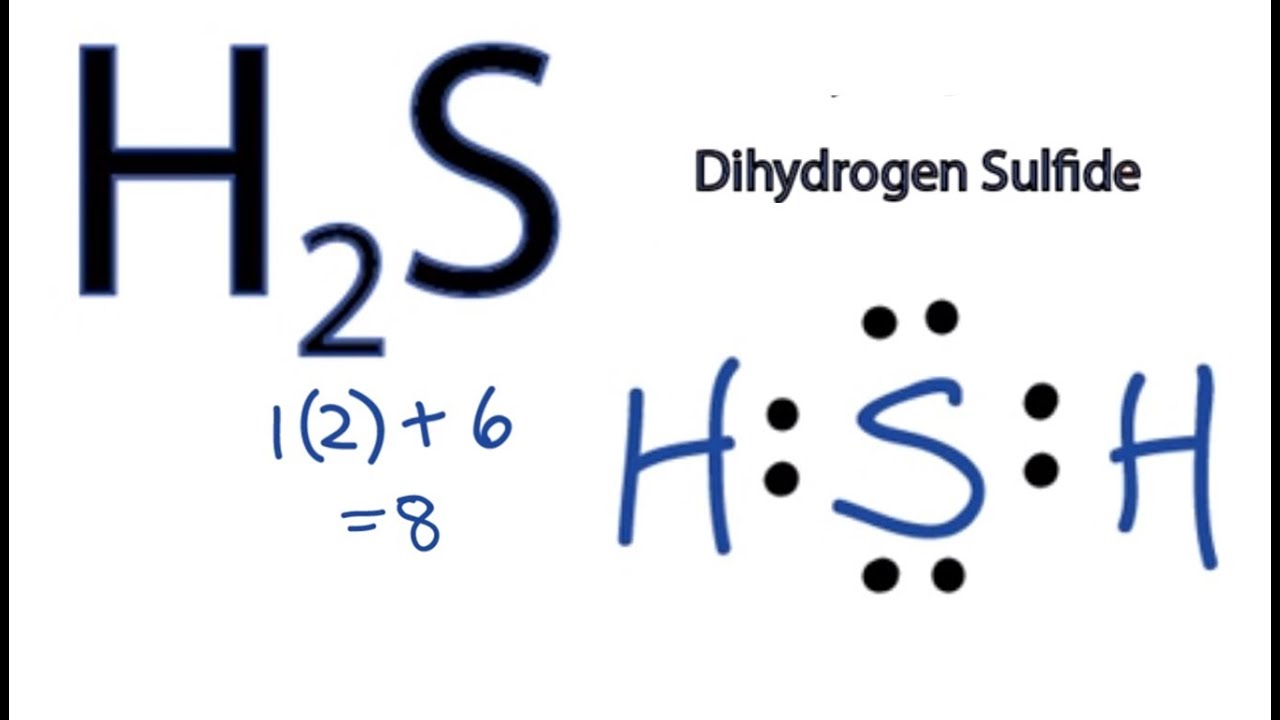
H2s Lewis Structure How To Draw The Dot Structure For H2s Youtube

Ni3 Lewis Structure How To Draw The Dot Structure For Ni3 Nitrogen Triiodide Youtube
0 comments
Post a Comment